
Sürmene Bıcak İmalatcısı.Süleyman Usta - Her model özel tasarım.Avcı bıçakları.imalatcısı.Süleyman Usta | Facebook
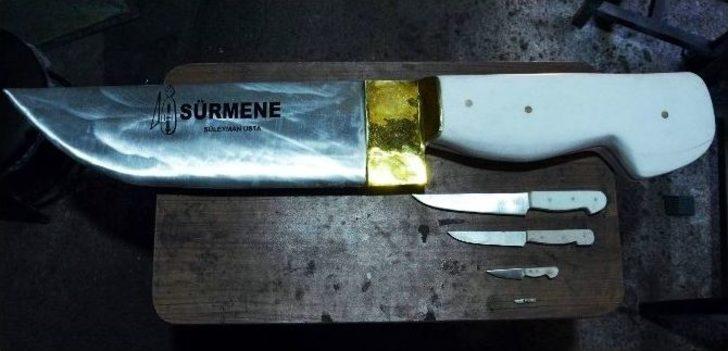
özel Haber) Sürmeneli Bıçak Ustaları Dünyanın En Küçük Ve En Büyük Bıçağını Yapma Konusunda İddialı - Trabzon Haberleri
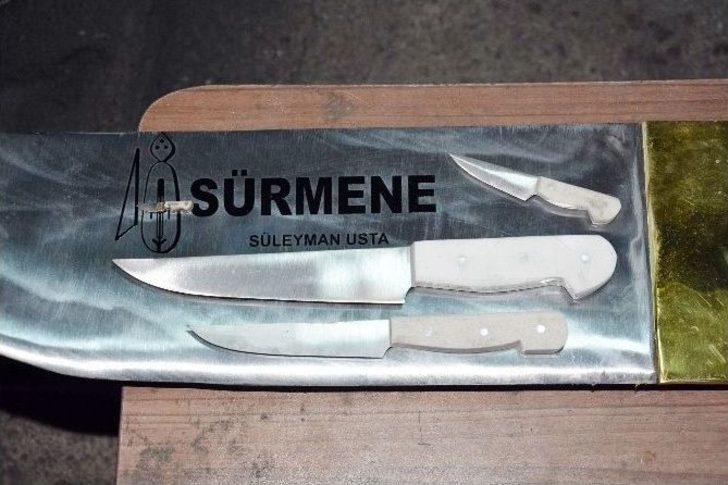
özel Haber) Sürmeneli Bıçak Ustaları Dünyanın En Küçük Ve En Büyük Bıçağını Yapma Konusunda İddialı - Trabzon Haberleri



















